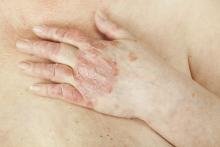
Safe and effective vaccines against covid-19 in rheumatic patients
Estefanía Fajardo
Vaccination against covid-19 is progressing in different countries and has allowed SARS-CoV-2 peaks to have a lower impact on hospitalization and deaths, compared to earlier peaks without the availability of the vaccines.
However, there are still many unresolved questions regarding safety, effectiveness, adverse effects, and other aspects in patients with autoimmune diseases, which is why different research groups have set themselves the task of carrying out these different studies and analyses to provide greater security and support in decision making.
This topic will also be addressed at the PANLAR 2022 Congress, in addition to the effects of the pandemic on health systems and the mental health of patients. What is the safety and efficacy of vaccines in Latin America and specifically in rheumatic patients in the region? These articles guide us in that line and aim, with evidence, to identify tools for physicians and information for patients in decision making.
One of the works focuses on a cohort from Argentina (ABS-1293) to describe the safety of SARS-CoV-2 vaccines in patients with rheumatic diseases and identify risk factors for adverse events and disease outbreaks after of vaccination.
The conclusions state that female gender, younger age, higher level of education, ChAdOx1 nCov-19 and mRAN-1273 vaccines, use of methotrexate and antimalarials were related to adverse events.
Another of this same line of safety and efficacy of vaccines against SARS-CoV-2 in patients with immune-mediated rheumatic and inflammatory diseases (ABS-1235) establishes that, in this cohort of patients vaccinated against SARS-CoV-2, the most used vaccines were Gam-COVID-Vac and ChAdOx1 nCoV-19. A quarter of the patients presented an adverse event and 5.1% presented SARS-CoV-2 infection after vaccination.
While another does the analysis in rheumatic patients under biological treatment (ABS-1236) in which 70 patients were included and it was determined that in them the covid-19 vaccine produces immunity in the vast majority of cases (initial response of 94, 3%), regardless of the disease, biological treatment and the type of vaccine administered.
Among patients with low levels of antibodies, the booster vaccine produced an increase to high levels in 82.6% of cases, and previous treatment with Rituximab could be related to the absence of development of SARS-CoV-2 neutralizing antibodies.
In this same direction, a work comparing the humoral response between patients with Systemic Lupus Erythematosus (SLE) and other autoimmune diseases was presented and the associated variables were analyzed (ABS-1332), establishing that vaccination with two doses of SARS-CoV-2 in patients with autoimmune rheumatic diseases presented a seroconversion rate of 70.2%.
Additionally, there were no differences in serological response between patients with SLE and other rheumatic diseases. Also, the levels of seroconversion and antibody titers were associated with the type of vaccine applied, with Sinopharm having the lowest response.
Continuing with lupus, the safety evaluation of the vaccines Bnt162b2, Sinovac and Chadox1 (ABS-1525) was presented, establishing that these are safe in patients with SLE, without serious adverse effects or worsening of disease activity.
Also, in this approach is the comparison of the immunogenicity of Pfizer/BioNTech, Sinovac and AstraZeneca vaccines in patients with SLE (ABS-1520), concluding that all three covid-19 vaccines after two doses induced a significant production of antibodies against the disease, which can improve protection against infection. However, the use of immunosuppressive therapy (cyclophosphamide and high doses of GCS) may significantly reduce this response.
Immune responses to SARS-CoV-2 vaccines in patients with rheumatic diseases (ABS-1163) were also reviewed in order to fully characterize the B and T cells immune responses elicited by mRNA vaccines in patients with rheumatic diseases. under immunotherapies, and identify which drugs reduce the immunogenicity of the vaccine.
In this work, it was established that patients with immune-mediated inflammatory and rheumatic diseases show impaired immunogenicity of the SARS-CoV-2 vaccine, which is variably reduced by immunosuppressants. Among the commonly used therapies, abatacept and B-cell depleting therapies show deleterious effects, while antitocins preserve immunogenicity. Also, the effects of cumulative doses of methotrexate and glucocorticoids on immunogenicity must be considered. Humoral and cellular responses are weakly correlated, but CD4 and CD8 are strongly correlated. Seroconversion alone may not reflect vaccine immunogenicity.
In addition to the review under complete schemes, another article (ABS-1253) analyzes the humoral and cellular immune response to the third vaccine in patients with rheumatoid arthritis who did not seroconvert after the primary two-dose regimen with inactivated or vector-based vaccines.
There was a total of 21 patients and in this cohort 90.5% presented detectable anti-SARS-CoV-2 IgG after a third dose. The use of abatacept was associated with a lower frequency of T-cell response.
Another of the topics addressed in these works was 'Sars-CoV-2 infection after vaccination in patients with rheumatic diseases in Argentina' (ABS-1303) in which a total of 1,350 patients from the SAR-COVID registry were included and it was established that only 5% of patients with RD vaccinated against SARS-CoV-2 had covid-19, most of them mild, and 25% were diagnosed after completing the scheme.
Finally, another pathway of the works presented is in line with the appearance of autoimmune diseases associated with vaccination. One of these was the new appearance of autoimmune diseases associated with covid-19 vaccines (ABS-1535), indicating that many adverse effects due to the different vaccines have been described; outbreaks of autoimmune diseases, and the new appearance of these pathologies (vasculitis, LUPUS, autoimmune encephalitis, among others).
In this cross-sectional study (there were 42 patients, 23 men, 19 women) and the mean time elapsed from vaccination to the appearance of symptoms was 9 days (1-63 days) and most patients (64%) began with symptoms at the second dose. It concludes that although vaccines are safe and effective, adverse effects such as neurological disorders, endocrinological and hematological autoimmune diseases may occur. Adenovirus technology vaccines were the most frequently associated.
As well as a systematic review of this topic (ABS-1548) with an electronic search that yielded 264 publications. A total of 150 articles were evaluated and 45 reports with 62 patients were included, concluding that, despite the increasing number of documented cases, the incidence remains low compared to the benefits of vaccination. Emphasizing that there is a lack of information in Latin America and strategies should be implemented to promote the notification of these cases in Latin American countries.
IMPACT ON HEALTH AND MENTAL HEALTH SYSTEMS
In 2020, the rapid evolution of the SARS-CoV-2 pandemic triggered a health emergency, applying mandatory social isolation measures. At the same time, it generated a major reorganization of the healthcare system in response to the growing demand, leading to the interruption and subsequent adaptation of training systems.
Therefore, the effects of the covid-19 pandemic environment on disease activity and access to health services in patients with rheumatoid arthritis (ABS-1403) were reviewed, indicating that patients with low baseline disease activity managed to access medical care during the pandemic, in addition to the observation of a reduction in the DAS28-ESR, possibly due to less exposure to common environmental stressors as a consequence of home isolation and social distancing.
In this sense, an analysis of the impact of the pandemic was generated in a group of rheumatology students from Argentina (ABS-1357). Of the 114 students contacted, 79 responded and it was determined that the pandemic affected them, limiting their practical and academic activities, and having to perform tasks unrelated to their training in more than half of them. Besides, they were able to learn new strategies to continue providing medical care to patients.
On the other hand, and in the mental health route, fear of covid-19 and its correlation with mental disorders in patients with autoimmune rheumatic diseases (ABS-1370) were reviewed, establishing that the factors associated with fear of covid-19 were female gender, treatment for mental disorders, and a diagnosis, while being divorced or widowed appear to be protective. Therefore, it is suggested that rheumatologists should identify these characteristics in order to offer adequate support.
Remember that the complete schedule of activities, with details of all the daily, can be reviewed here. In addition, you may access all the papers presented in the conference abstract book (1).
REFERENCES
- JCR: Journal of Clinical Rheumatology: July 2022 - Volume 28 - Issue S1 - p S1-S95. Available at https://journals.lww.com/jclinrheum/Citation/2022/07001/PANLAR _Abstracts_2022.1.aspx